Mechanochemical reactions encompass a glut of chemistry that follows the crushing of rocks and minerals. Reactions of water with crushed rock are currently changing our understanding of the energy available to life in extreme environments. These reactions can produce H2O2, H2, CO2, and maybe even methane, providing a range of useful molecules for life. The main way that this happens is from the breakage of bonds between silicate rocks, forming highly reactive radicals (mainly SiO• and Si•) on the surface of the rock that react with water (Eq. 1).
Eq. 1 ≡Si–O–Si≡ → ≡Si–O• + ≡Si•
The issue is that nature has countless rock-crushing methodologies (grinding, smashing, even squashing), there are many rocks, and each has its own complex mineral assemblage and structure. Even minor differences can alter the types of surface defects that form, and each defect changes and reacts in diverse ways depending on an innumerable number of factors.
I came into this research project with an undergraduate degree in zoology and an interest in astrobiology. I was excited about Dr Jon Telling’s mechanochemistry research at Newcastle university as it related to potential energy sources for alien life living on icy moons such as Enceladus and Europa. So, I joined his team as a masters student studying environmental geoscience.  Based on my interest in icy moons, we began by following on from Jon’s glacial research on sources of hydrogen from low-temperature water rock reactions. We were also interested in how rapid glacier movements might affect subglacial water rock reactions, supposed ‘stick-slip’ glacier motion. To mimic subglacial conditions, we set up vials with crushed granite, added anoxic water, and ‘flash’ heated the vials to simulate rapid glacier ‘slips’.
Based on my interest in icy moons, we began by following on from Jon’s glacial research on sources of hydrogen from low-temperature water rock reactions. We were also interested in how rapid glacier movements might affect subglacial water rock reactions, supposed ‘stick-slip’ glacier motion. To mimic subglacial conditions, we set up vials with crushed granite, added anoxic water, and ‘flash’ heated the vials to simulate rapid glacier ‘slips’.
Mechanochemical reactions generate H2 from reactions of water with Si• (Figure 1c). The obvious expectation would be that increasing temperatures would increase the rate of a chemical reaction, and thus, more H2 would be generated from mechanochemical reactions at higher temperatures. This has been observed in the literature (e.g. (Parkes et al., 2011). Our experiments with granite, however, reversed the trend (see Figure 1a; 168 h).
.png)
While initially perplexed by this result, a paper from 1982 (Kita et al., 1982) was our saving grace. We had replicated their results, albeit at a lower temperature. Kita et al. explained that the trend was caused by the high-temperature activation of SiO•, which consumes single hydrogen atoms before they can combine to form H2 (Figure 1b). At lower temperatures, H2 is produced because SiO• is inactive (Figure 1c), but at higher temperatures, SiO• becomes reactive and absorbs the hydrogen atoms before they can form H2 (Figure 1d). Since 1982, SiO• has also been implicated in the generation of oxidants and oxygen (Gill-Olivas et al., 2021), so maybe if we were activating this radical, we were also generating oxidants. With just a few months left to collect data for my masters project, we decided to ditch the subglacial aspect of this project, and pursue these interesting high temperature results. This time the experiments were targeting subseafloor fractures in tectonically active regions where life may have originated and the earliest life forms may have lived.
We set up the vials as before, but this time with crushed rocks targeting oceanic crust (basalt and peridotite) along with the original interesting rock type, granite (targeting continental crust; Figure 2). The vials were heated to higher temperatures (60, 80, 105 and 121 °C) and kept there this time for an hour, a day, or a week. We measured the generation of H2 and H2O2 from reactions of water with the crushed rocks, all under extremely well controlled oxygen conditions (<0.1% O2).
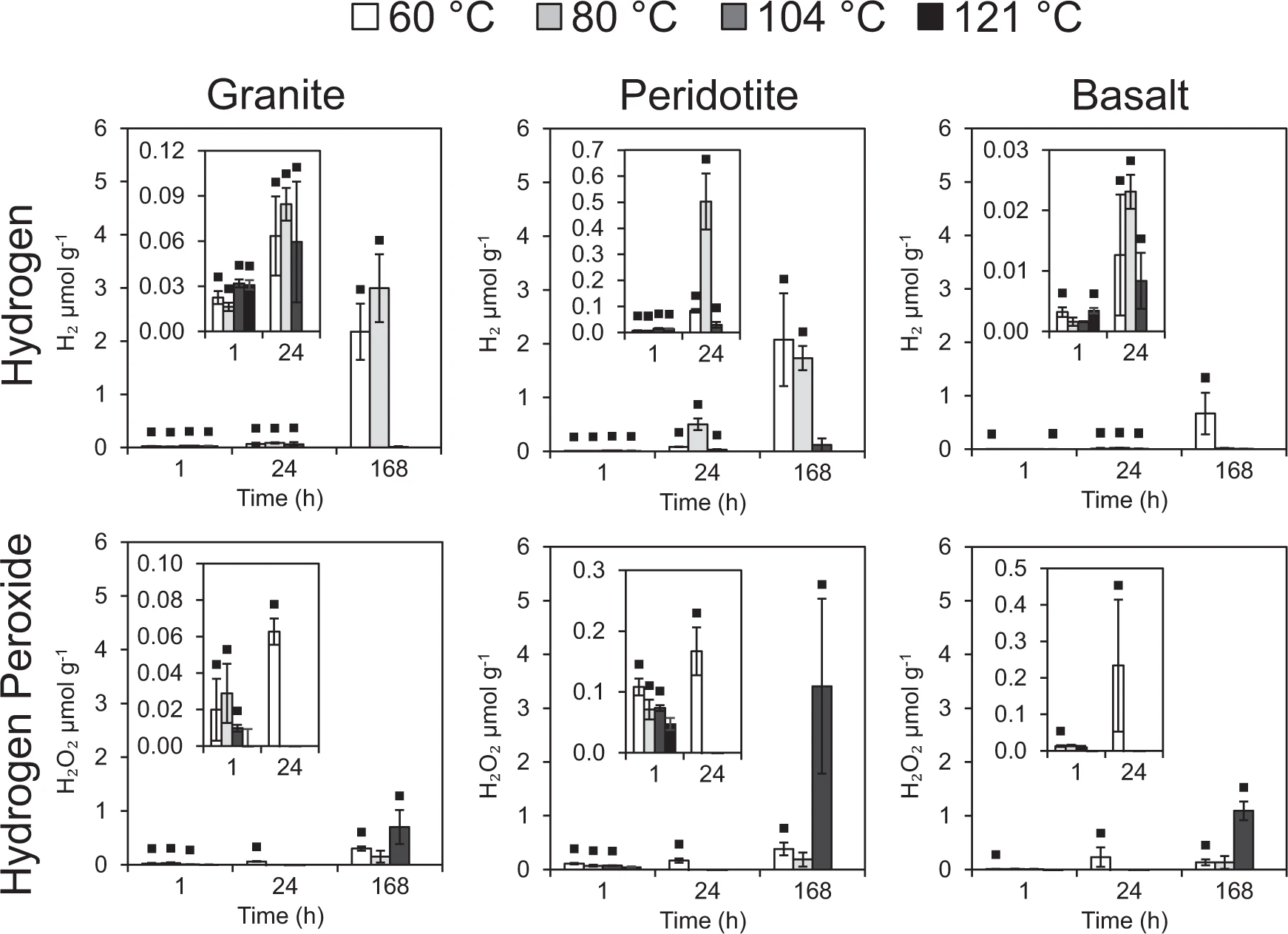
As before, the H2 production stopped after a week at the highest temperature (121 °C), but this time much more dramatically than before and with all three rock types (Figure 2). At the same temperature and time point, we generated hydrogen peroxide (Figure 2). A mechanism involving the adhesion of O2 to the surface of the silicates was ruled out because O2 was not plentiful enough to explain the generation of the SiOO• radicals necessary to generate it. This implicated SiO• in the generation of our oxidants. This lack of oxygen requirement was a key factor, as it means that oxidants could be generated in anoxic environments, such as before the great oxidation event (figure 3). Note that hydrogen peroxide (a very potent oxidant itself) can easily be broken down to oxygen and water abiotically or through enzyme activity.

These experiments were all conducted in the lab. It can be hard to make the leap from a borosilicate vial to subsurface fractures in the early Earth. So, we looked to the wider literature for evidence that this oxidant production mechanism really exists. The first evidence was that lithoautotrophic methanogenesis, a microbial metabolism utilising H2 and CO2 for energy, stops entirely in subseafloor sediments when temperatures reach 80 °C (Heuer et al., 2020). Perhaps this is because the source of their H2 is mechanochemical reactions, and in line with our experiments, SiO• inhibits the formation of H2 at temperatures above 80 °C (Figure 2). This was a promising grounding of our research and indicated that the first part of our mechanism may operate in natural environments and have significant impacts on microbial communities.
Looking now to ancient Earth, LUCA (last universal common ancestor) is an ancient microbial organism that all living things are thought to have evolved from. It likely lived in hot environments (was a hyperthermophile; (Weiss et al., 2016), similar to the conditions that our data indicate oxygen might have formed in the ancient Earth. This could be a coincidence. However, genetic reconstructions of LUCA’s genome have found genes for cycling H2O2 and O2 for energy (Weiss et al., 2016, Ślesak et al., 2012). This finding was dismissed as an artefact of later evolution, but considering our data, this can now more easily be explained by the presence of tectonic H2O2 and O2 created in the very environments that LUCA likely existed. Estimates about the onset of plate tectonics also seem to line up quite well with the time when life first originated (Hawkesworth et al., 2020), revealing O2 (and hydrogen which is key for creating organic molecules) from mechanochemical reactions as a potential energy source for the origin of life. The implications of this research are, thus, not only crucial for the early evolution of life but the origin of life itself.
The research project is continuing with a new grant awarded to Dr Jon Telling and the CERBERUS team based at Newcastle University (https://research.ncl.ac.uk/cerberus/), no doubt with many more exciting discoveries to be made. The project was conducted as part of the Environmental Geoscience MRes course at Newcastle University (https://www.ncl.ac.uk/postgraduate/degrees/4867f/). Read the paper here: https://www.nature.com/articles/s41467-022-32129-y
References
GILL-OLIVAS, B., TELLING, J., TRANTER, M., SKIDMORE, M., CHRISTNER, B., O’DOHERTY, S. & PRISCU, J. 2021. Subglacial erosion has the potential to sustain microbial processes in Subglacial Lake Whillans, Antarctica. Communications Earth & Environment, 2, 1-12.
HAWKESWORTH, C. J., CAWOOD, P. A. & DHUIME, B. 2020. The evolution of the continental crust and the onset of plate tectonics. Frontiers in earth science, 8, 326.
HEUER, V. B., INAGAKI, F., MORONO, Y., KUBO, Y., SPIVACK, A. J., VIEHWEGER, B., TREUDE, T., BEULIG, F., SCHUBOTZ, F. & TONAI, S. 2020. Temperature limits to deep subseafloor life in the Nankai Trough subduction zone. Science, 370, 1230-1234.
KITA, I., MATSUO, S. & WAKITA, H. 1982. H2 generation by reaction between H2O and crushed rock: an experimental study on H2 degassing from the active fault zone. Journal of Geophysical Research: Solid Earth, 87, 10789-10795.
PARKES, R. J., LINNANE, C. D., WEBSTER, G., SASS, H., WEIGHTMAN, A. J., HORNIBROOK, E. R. & HORSFIELD, B. 2011. Prokaryotes stimulate mineral H2 formation for the deep biosphere and subsequent thermogenic activity. Geology, 39, 219-222.
ŚLESAK, I., ŚLESAK, H. & KRUK, J. 2012. Oxygen and hydrogen peroxide in the early evolution of life on earth: in silico comparative analysis of biochemical pathways. Astrobiology, 12, 775-784.
WEISS, M. C., SOUSA, F. L., MRNJAVAC, N., NEUKIRCHEN, S., ROETTGER, M., NELSON-SATHI, S. & MARTIN, W. F. 2016. The physiology and habitat of the last universal common ancestor. Nature microbiology, 1, 1-8.







Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in